Today's Patreon-fueled shout-out is for the Plant Northern Piedmont Natives Campaign, an initiative that wants you to grow native plants in yards, farms, public spaces and gardens in the northern Piedmont. Native plants provide habitat, food sources for wildlife, ecosystem resiliency in the face of climate change, and clean water. Start at the Plant Northern Piedmont Natives Facebook page and tell them Lonnie Murray sent you!
You too can get a Patreon-fueled shout-out for a nonprofit or a cause for $25 a month. That helps keep this experiment going, and helps you get the word about something you care about. Consider doing so today!
*
We’re now nine and a half months into the pandemic, and there are signs that one day this will all be over. This daily newsletter has its origins in a podcast I began in the middle of March to document the experience. This installment sort of doubles up as installments of both products, as our only story today is about the pandemic and the roll-out of the two vaccines that are approved so far.
Over a million people in America have been vaccinated in the past eleven days according to a press release from the Centers for Disease Control. However, cases continue to rise across the country, with over 195,000 reported yesterday. Over 300,000 people have died so far, and we are all watching to see if there is another surge in new cases related to Christmas, just as there has been a spike following Thanksgiving.
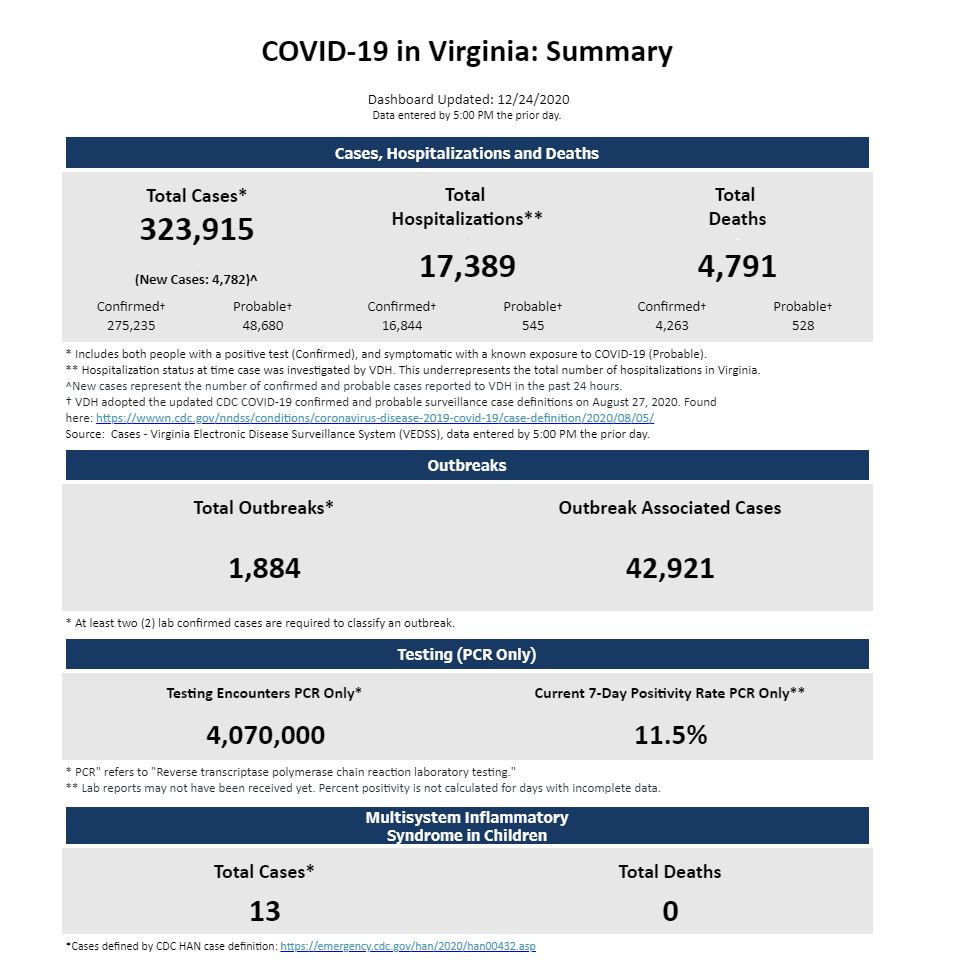
Today in Virginia, the Department of Health reports an additional 4,782 new cases, the second record day in a row for the Commonwealth. In the Blue Ridge Health District, there are another 104 cases. Since Thanksgiving there have been just over 1,800 cases in the district, or about a quarter of the total number of cases so far.
There have been 90 deaths in the District, which consists of the city of Charlottesville as well as the counties of Albemarle, Fluvanna, Greene, Louisa and Nelson.
At the center of this pandemic, two area hospital systems have borne the brunt of treating patients and administering the many tests that have been conducted since March. There have been over 175,000 tests and the seven-day average of positive tests is currently 5.6 percent.
But now, a new set of statistics will begin to be reported as more people receive a vaccine. Among the first locally are medical personnel at the University of Virginia Health System. As of Wednesday, nearly 1,600 had received the shot.
Wendy Horton, chief executive officer of the University of Virginia Health System, said they are continuing to vaccinate employees.
“We’re in the process of getting everyone getting scheduled for an appointment to receive a vaccine in the teams,” Horton said. “We currently have about 4,000 individuals signed up for an appointment. And our goal as a health system is to try to vaccinate all of UVA Health by the end of March and that would include the second dose. As you know, these vaccinations require a second dose.”
Horton said UVa has been able to get 380 additional doses of the Pfizer vaccine due to a surplus amount in each vial. UVA also received a first shipment of 2,500 doses of the Moderna vaccine and will switch to that as soon as the Pfizer supply runs out.
Health workers and those who live in long-term care facilities are the first to receive the vaccines. Dr. Costi Sifri is an infectious disease expert who has been leading up UVa’s efforts.
“We’ve been incredibly gratified and excited about this opportunity to provide vaccines for our team neighbors and they’re certainly responding with their excitement as well,” Sifri said. “For many of us, this is some of the most important work that we’ve done. One of my colleague said it’s the most important work she’s even done in her entire career. “
But what next now that there’s a vaccine? There are still a lot of unknowns. At the briefing, UVA Health spokesman Eric Swensen relayed questions from media folks. Let’s listen in.
Swensen: “Can you transmit the virus once you have the vaccine?”
Sifri: “That’s an excellent question and something we’re still trying to learn about. The outcomes from the clinical trials were whether it prevented disease and secondarily whether it prevented severe disease. Whether it prevents virus replication, the presence of virus and the state of being a virus carrier is not known yet.
“There is some suggestion from one of the clinical trials with the Moderna vaccine that perhaps it does reduce the amount of live virus, so that’s going to be a focus as we move forward. So more importantly, because we don’t know that answer, what the instructions are from the CDC, and certainly what we are promoting that even if our team members are vaccinated we still need to maintain the practices to prevent transmission of the virus. Wearing the mask, maintaining social distance, those types of things.
The second question was how UVA is responding to a climb in cases at the hospital. The COVID tracker shows 60 cases at the hospital today. What happens if that number rises with the increased number of cases across the region?
“We’re feeling really overall well prepared,” Horton said. “We have an amazing team that’s really come together to support the needs of the community and the patients that we serve. Just a couple of updates. We have adequate PPE and adequate testing right now and our approach has been for the last several weeks is to really care for both COVID and non-COVID patients and that really means the same. And so we’re checking in multiple times a day to make sure we’re adjusting as needed the operations to care for all of the patient populations.”
“The majority of patients are really being cared for in an ambulatory or outpatient setting both through the COVID clinic and many programs including our home-monitoring program,” Horton continued. “On the in-patient side, we have seen that uptick in our COVID in-patients, and just wanted the team to know that we do have a response plan and there are a series of steps and lever that we monitor every day and we’re titrating and making different plans each and every other day to meet the various needs.”
In late November, the in-house publication posted a Q&A on this topic that I’d recommend reviewing. Let’s continue to keep an eye on this. In the meantime, Horton said the potential for concern does not relate to supplies or infrastructure.
“Our rate limiting step will be our staffing and not really our facility at this point in time so that’s the piece we’re looking at, we’re looking at the skill set and the patient care needs and making sure we’re synching those up and working together as a team to move people around as needed,” Horton said.
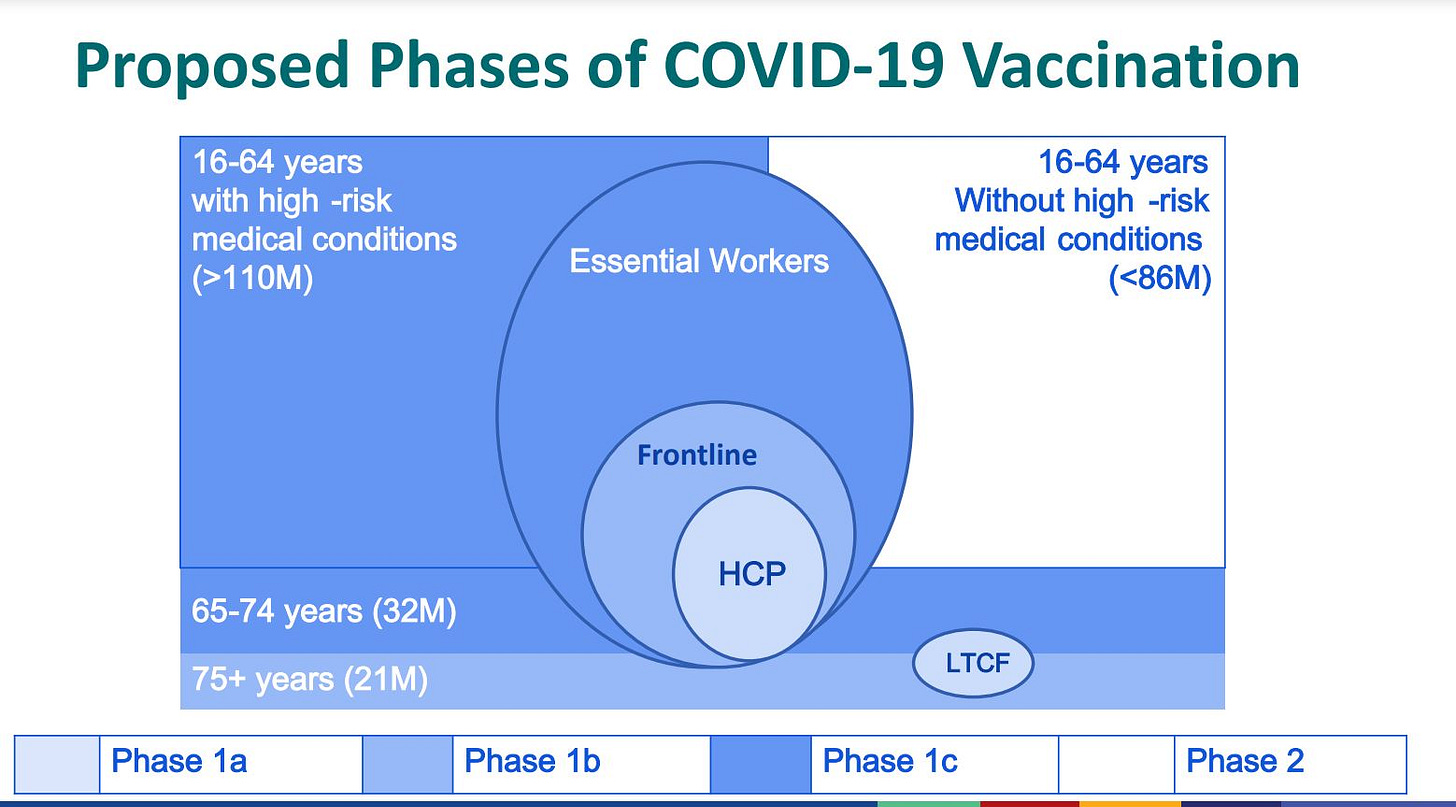
Now, back to vaccines. We’re currently in Phase 1A of the CDC’s recommended prioritization of the limited number of doses. Phase 1B and Phase 1C are next, with 1B for people 75 and over as well as frontline essential workers. Phase 1C will be for people 65 and over, people with medical conditions, and other essential workers.
“It remains to be seen when those occur, but they will occur under the guidance of the health department who will be following the CDC guidance we would anticipate,” Sifri said. “I would predict that they would occur sort of in an overlapping fashion. Certainly that’s what been discussed but I think we need to wait to exactly see how that is rolled out.”
Let’s hear some more questions.
Swensen: “Have there been any complications or reactions to anyone who has received the vaccine so far?”
Sifri: “So, no, there have been no serious side effects. People have had local pain and discomfort has been widely reported. We have heard reports of three people who have had complaints after getting the vaccine after they were sitting for their observation period after they got the vaccine, two people with nausea. One person that felt light-headed, but that’s been it so far. It’s fairly well tolerated. I got my vaccine yesterday. Happy to have been able to get mine.”
Dr. Sifri and everyone else who has had a first vaccine will have to get a second dose in a few weeks.
Here’s another question.
Swensen: “Why are two doses required for each of these vaccines? Why do they need to be given roughly 21 days apart?”
Sifri: “This is not uncommon in terms of other vaccine series that you essentially give a first shot to prime the immune system and then a second vaccine to boost the immune system, so there’s a priming and a boosting phenomenon. This is based on the vaccine evaluation when it was going through clinical trials. It started in the laboratory and then moved into those early clinical phases. The scientists that helped develop these vaccines understood that part of immune response. They are immune experts and vaccinologists and so that was how that was determined. That’s also why they are separate by three weeks for the Pfizer vaccine and four weeks for the Moderna vaccine. Again, analysis performed during the clinical evaluations of these vaccines, that was what was determined to lead to the best boosting phenomenon for the two respective vaccines.”
Dr. Sifri said there are two hundred other vaccine candidates in some state of development.
Sifri: “In terms of vaccines that are in late phase clinical trials, there are five that are sort of towards the end of that process. The two that have received authorization we’ve talked about are Moderna and Pfizer. The three that are currently underway and we’re waiting to hear more information about them are based on two different platforms. One are what we call vector-based vaccines so two vaccines that are based on adeno virus platforms so that the vaccine is contained within a non-replicating virus that can be given to people. The two companies are Astra Zeneca and Johnson & Johnson.
“The interesting thing about one of those products is that the Johnson & Johnson is that part of the clinical trial involves a single dose of the vaccine. That would certainly be exciting if that shows to be safe and efficacious similar to the ones we have on hand now just because it would be much easier to administer.
“The fifth vaccine, and again, there are some waiting in the wings right behind it, is a protein-based vaccine and that’s by a company called NovoVax and the platform is rather similar type of concept for what is used for hepatitis-B vaccinations.”
This time last year to most of us, the coronavirus was something far away, a news story from China. Now, over a million Americans have received a vaccine. The next question asked sought to put this in historical perspective.
Swensen: “How does the roll out of the COVID vaccine compare with other vaccines from other major pandemics, diseases, in terms of the length of time it took to get this vaccine developed and actually begin the vaccination process?”
Sifri: “This is a landmark. It’s unprecedented to have gone from the identification of a novel pathogen, a novel virus. Its genome was published on January 10. To have eleven months later to have an authorized, actually two authorized vaccines that are highly efficacious and safe. It’s clearly a landmark of science. The question is how fast is that compared to previous. I think it’s a couple years faster that what has been seen typically. It is under unique circumstances, though, however. We have a worldwide pandemic with high amounts of disease. That actually has leant itself for the development of these vaccines because we can get to the end points and see if the vaccine was effective rather quickly compared to other vaccines that are in development and may take years to get through because it takes a while to accrue the patients and see what the outcomes were. It is unprecedented but it was clearly the best opportunity we had to change the course of this pandemic.
“Our previous experience that is somewhat akin to this was the development of the swine flu vaccine in 2009 and its night and day different compared to that. In 2009, the development of the vaccine was very flow during the course of that pandemic even though the technology was there and we had a very challenging time getting the vaccine out and certainly what the hope is that the process will be much brisker this time.”
Swensen: “I think that’s sort of where this question comes from. This, as you said, this amazingly fast turnaround for the development and delivery of this vaccine, what does that mean for the future kind of dealing with down the in the future, knowing this probably won’t be the last novel pathogen or virus? What will this mean for how we’re able to tackle future potential pandemics like this?”
Sifri: “The learning that we’ve had from this pandemic is important learning that we can’t forget. We’ve now had demonstration that we’ve had platforms that were ready to develop vaccines that were ready to go and by using those platforms, we were able to develop what appears to be at least two effective vaccines and hopefully more in very rapid period of time. That from a scientific standpoint, the ability to have those platforms to plug and play with new emerging pathogens is quite exciting.
“You know, every pathogen, every new emerging pathogen will have challenges, but you’re right, Eric, this is not the last time we’ll see this and hopefully we’ll be able to capitalize on the knowledge that we’ve gained from this pandemic to confront future challenges.”
Thanks for listening and thank you for your support. Here are some ways you can help ensure I can keeping this work into 2021 and beyond!
Support my research by making a donation through Patreon
Sign for a subscription to Charlottesville Community Engagement, free or paid















Share this post